Introduction: Severe hemophilia A (HA) in females is incredibly rare particularly if complicated by a high-titer factor VIII (FVIII) inhibitor. We report, to the best of our knowledge, the first-of-its-kind use of emicizumab (emi) ante/post-partum, its effect on the newborn, and its excretion in breast milk.
Baseline details: 29-year-old-female was diagnosed with severe HA at age 1 following prolonged bleeding from a venipuncture site. She had no family history of HA. One stage FVIII activity (OSA) was less than 1%. She was compound heterozygous for F8 type 1 and type 2 intron 22 inversions. At 21 months of age, she developed a high-titer inhibitor (peak: 35 Bethesda unit[BU]). She was not treated with immune tolerance induction and had remained on factor eight inhibitor bypassing activity (FEIBA) as prophylaxis until transferring to our center. She was transitioned to monthly emi prophylaxis and recombinant FVIIa (rFVIIa, NovoSeven) for breakthrough bleeding at age 26. She was medically advised against pregnancy due to unclear safety profile of emi in pregnancy and theoretical concerns for an increased risk of bleeding and thrombotic complications associated with the use of bypassing agents in the peripartum setting. Nevertheless, she desired pregnancy and continued on emi prophylaxis every 4 weeks during her pregnancy.
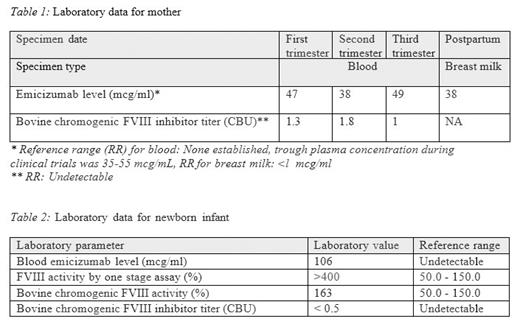
Clinical course in pregnancy: She had an uncomplicated antepartum course and did not require rFVIIa for breakthrough bleeds. Blood emi levels and FVIII inhibitor levels were measured in each trimester of pregnancy (Table 1). Her labor was induced with oxytocin while on rFVIIa and tranexamic acid (TXA) support. She had a spontaneous, unassisted vaginal delivery immediately complicated by post-partum hemorrhage resulting in estimated blood loss of 1100cc. She did not require blood transfusion. She continued on 90 mcg/kg rFVIIa support every 2 hours, gradually prolonging the dosing interval by 2 hours every 24 hours (q2, q4, q6, etc.), and experienced no further in-hospital bleeding. She delivered a healthy female newborn. The infant developed cephalhematoma but transcranial ultrasound was negative for intracranial bleeding. Cord blood was collected at the time of delivery and sent for emi level, FVIII activity and FVIII inhibitor titer (Table 2). FVIII inhibitor assay with bovine reagent (CBU) was performed using chromogenic substrate assay (CSA) after pre-analytical heat-inactivation of patient plasma. The infant's CSA FVIII level was measured using a bovine chromogenic FVIII assay, and FVIII was also assayed using a one-stage APTT-based assay. Mother opted to breast-feed the infant. Emi level was measured in breast milk (Table 1).
Conclusion: In this highly unusual case, we demonstrate that emi crossed the placenta and is excreted in breast milk. We also demonstrate that the maternal FVIII inhibitor did not cross the placenta into the newborn. Drawing conclusions on the safety of emi despite placental and breast milk transmission and transmissibility of inhibitor based on a single case would be premature. The conglomeration of several rarities in a single case renders our report unique and insightful and provides a baseline experience for future cases.
Disclosures
Pruthi:Instrumentation Laboratories (Werfen): Consultancy, Honoraria; HEMA biologics: Consultancy, Honoraria; Bayer Healthcare AG: Consultancy, Honoraria; Genentech Inc.: Consultancy, Honoraria; CSL Behring: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal